STEP 1: Initial assessment of a given patient that yields suspicion for Prostate Cancer and a biopsy of the prostate is indicated. This suspicion is risen by: clinical data - family history, ethnicity risk factors, along with elevated PSA levels, velocity, abnormal rectal exam - or by newer molecular risk assessment such as 4K score, Phi Index- etc.
STEP 2: The patient undergoes a Multi-parametric MRI. Thorough assessment of the MRI is performed in order to identify any potential lesion or ill process
STEP 4: Either using our local anesthesia technique or under sedation or regional anesthesia the biopsy can be performed employing a trasperineal technique "real-time" fusion or under a transrectal approach either under real-time or cognitive fusion. The advantajes of a transperineal approach include a lower risk for infections as the rectal mucosa is avoided and superior precision in focal treatment for those eligible.
FOCALYX® Bx: Step by Step
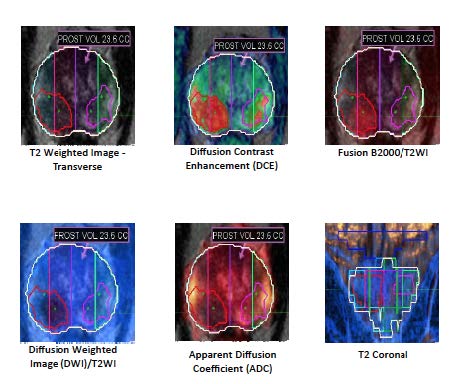
STEP 3: Our team reviews the Multi-parametric MRI and identifies any areas of interest - Focalyx Dx - this process requires of specific software and using proprietary algorithms anatomic landmarks such as prostate, seminal vesicles and the urethra are identified along with any significant lesion that has a >50% risk of harboring cancer cells.
The fusion software allows the planning of the MRI/Ultrasound Fusion Prostate Biopsy. The silhouettes of the prostate, its lesions and the planned sampling will be fused with the ultrasound prostate imaging, proving a GPS of the lesions and samples areas.